A ‘sulfonyl-azide-free’ (SAFE) aqueous-phase diazo transfer reaction for parallel and diversity-oriented synthesis†
Abstract
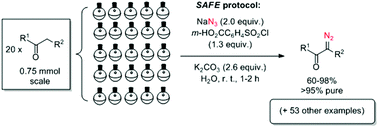
Diazo transfer reactions are notoriously associated with the use of potentially explosive sulfonyl azides. The first ‘sulfonyl-azide-free’ (SAFE) protocol for producing diazo compounds from their active-methylene precursors via the Regitz diazo transfer reaction was developed and has displayed a remarkable substrate scope. It can be applied to generating arrays of diazo compounds for further evolution via combinatorial chemistry and a range of scaffold-generating transformations.
- This article is part of the themed collection: Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...