Base-enhanced electrochemical water oxidation by a nickel complex in neutral aqueous solution†
Abstract
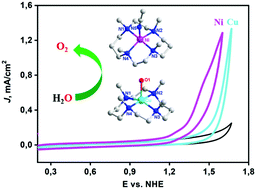
A nickel complex, [Ni(TMC)(CH3CN)](NO3)2 (TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane, 1), was found to be an efficient water oxidation catalyst in pH 7 phosphate buffer. It exhibits pseudo first order kinetics in electrochemical water oxidation with a catalytic rate of 9.95 s−1, the highest rate for nickel WOCs at neutral pH. Complex 1 also shows superior catalytic activity with respect to that of a copper analogue, [Cu(TMC)(H2O)](NO3)2, under the same conditions. Kinetic studies indicate a more than one order of magnitude rate acceleration with the added bases due to an atom-proton transfer (APT) pathway.


 Please wait while we load your content...
Please wait while we load your content...