A nickel(ii)-catalyzed asymmetric intramolecular Alder-ene reaction of 1,7-dienes†
Abstract
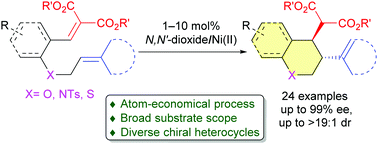
A highly diastereo- and enantioselective intramolecular Alder-ene reaction with an alkene as the enophile has been developed by using a chiral N,N′-dioxide/nickel(II) complex as the catalyst. This protocol provides a facile route towards the synthesis of diverse 3,4-disubstituted chroman, tetrahydroquinoline, piperidine and thiochroman derivatives in high yields with good to excellent diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...