Catalytic enantioselective Hosomi–Sakurai reaction of α-ketoesters promoted by chiral copper(ii) complexes†
Abstract
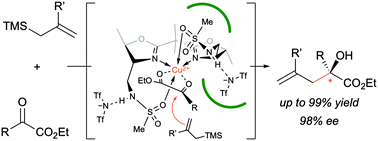
A catalytic enantioselective Hosomi–Sakurai reaction of α-ketoesters has been developed. A copper(II) complex with a chiral bis(oxazoline) ligand bearing methanesulfonamide groups shows excellent catalytic activity to give α,α-disubstituted α-hydroxyesters in high yields with high enantioselectivities. This is the first successful method for the catalytic enantioselective 1,2-addition of α-ketoesters with allylic silanes.


 Please wait while we load your content...
Please wait while we load your content...