An untethered C3v-symmetric triarylphosphine oxide locked by intermolecular hydrogen bonding†
Abstract
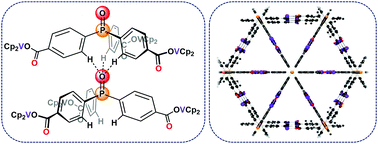
A para-substituted triphenylphosphine oxide with terminal vanadocene centers has been prepared and is, to our knowledge, the first example of an untethered C3v-symmetric triarylphosphine oxide in the solid state. Crystallographic and DFT studies suggest this locked conformation is due to intermolecular H-bonding interactions. Electrochemical measurements suggest these interactions may persist in solution. A monometallic variant, adopting the standard C3 propeller geometry, has also been synthesized for comparison.


 Please wait while we load your content...
Please wait while we load your content...