Dual nickel- and photoredox-catalyzed reductive cross-coupling of aryl vinyl halides and unactivated tertiary alkyl bromides†
Abstract
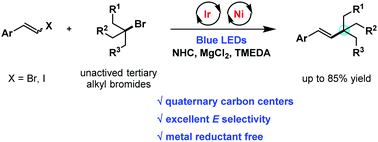
A novel reductive cross-coupling of aryl vinyl halides and unactivated tertiary alkyl bromides has been realized via photoredox/nickel dual catalysis to produce vinyl arene derivatives bearing all-carbon quaternary centers with excellent E-selectivity. A stoichiometric metal reductant could be avoided by employing commercially available N,N,N′,N′-tetramethylethylenediamine (TMEDA) as the terminal reductant.


 Please wait while we load your content...
Please wait while we load your content...