Benzyne-mediated trichloromethylation of chiral oxazolines†
Abstract
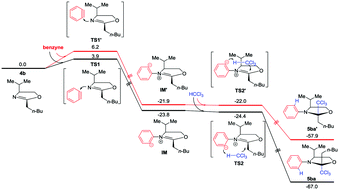
A three-component reaction between benzyne, oxazolines and chloroform was developed for the synthesis of trichloromethylated chiral oxazolidines. Benzyne not only serves as an electrophile towards oxazolines but also acts as a base for the deprotonation of chloroform. The dual functions of benzyne enable the trichloromethylation of chiral oxazolines and thus construct chiral N,O-quaternary stereocenters.


 Please wait while we load your content...
Please wait while we load your content...