Cryptand-imidazolium supported total synthesis of the lasso peptide BI-32169 and its d-enantiomer†
Abstract
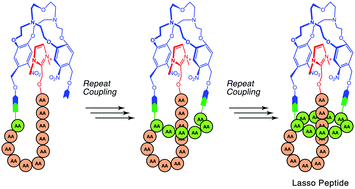
Lasso peptides are attracting increasing attention due to their broad range of biological activities. The knot topology of lasso peptides, which contains an isopeptide bond-bridged macrocycle threaded by its C-terminal tail, has been proven to be an important structural feature for their bioactivities. The preparation of lasso peptides has been achieved by biosynthetic methods; nevertheless, a chemical synthesis of lasso peptides has not been described so far. Herein, a cryptand-imidazolium complex is designed as a multi-linker support and applied in the chemical synthesis of the lasso peptide BI-32169. Furthermore, the chiral switching of the support and the introduction of D-amino acids enable the synthesis of the D-enantiomer of BI-32169, which shows not only a strong glucagon receptor antagonist activity, but also a much higher enzymatic stability compared to the L-lasso peptide.


 Please wait while we load your content...
Please wait while we load your content...