Enantioselective iridium-catalyzed carbonyl isoprenylation via alcohol-mediated hydrogen transfer†
Abstract
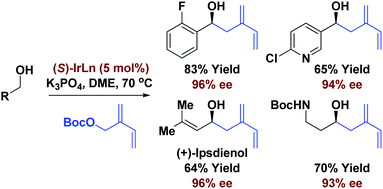
Highly enantioselective iridium catalyzed carbonyl (2-vinyl)allylation or “isoprenylation” is achieved via hydrogen auto-transfer or 2-propanol-mediated reductive coupling from primary alcohol or aldehyde reactants, respectively. Using this method, asymmetric total syntheses of the terpenoid natural products (+)-ipsenol and (+)-ipsdienol were achieved.


 Please wait while we load your content...
Please wait while we load your content...