Unravelling nucleophilic aromatic substitution pathways with bimetallic nucleophiles†
Abstract
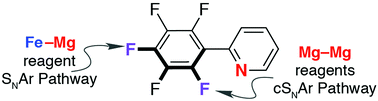
The reaction of a metal complex containing a polar Fe–Mg bond with 2-(pentafluorophenyl)pyridine leads to selective C–F bond activation. A stepwise SNAr mechanism involving attack of the bimetallic nucleophile on the electron-deficient aromatic ring has been identified by DFT calculations. Despite the long and rich history of metal anions in organic synthesis, this is the first time the SNAr mechanism has been elucidated in detail for metal-based nucleophiles.


 Please wait while we load your content...
Please wait while we load your content...