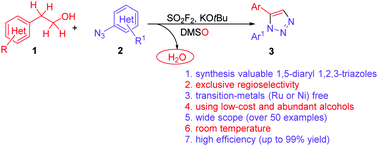
Transition-metal-free regioselective construction of 1,5-diaryl-1,2,3-triazoles through dehydrative cycloaddition of alcohols with aryl azides mediated by SO2F2†
Abstract
A novel, simple and practical method for mild, efficient, cost-effective and regioselective synthesis of highly valuable 1,5-diaryl-1,2,3-triazoles was achieved through dehydrative annulation of readily available alcohols with aryl azides. The reaction proceeded at room temperature, without any metal catalysts, exhibiting excellent compatibility to a large variety of functional groups (>50 examples), resulting in up to quantitative yields.


 Please wait while we load your content...
Please wait while we load your content...