Computational and experimental studies on copper-mediated selective cascade C–H/N–H annulation of electron-deficient acrylamide with arynes†
Abstract
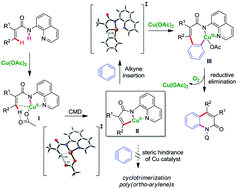
An efficient and convenient copper-mediated method has been developed to achieve direct cascade C–H/N–H annulation to synthesize 2-quinolinones from electron-deficient acrylamides and arynes. This method highlights an emerging but simple strategy to transform inert C–H bonds into versatile functional groups in organic synthesis to provide a new method of synthesizing 2-quinolinones efficiently. Mechanistic investigations by experimental and density functional theory (DFT) studies suggest that an organometallic C–H activation via a Cu(III) intermediate is likely to be involved in the reaction.


 Please wait while we load your content...
Please wait while we load your content...