Biodistribution and toxicological evaluation of micron- and nano-sized erythrocyte-derived optical particles in healthy Swiss Webster mice†
Abstract
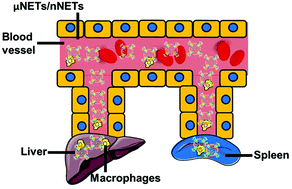
Particle-based systems provide a capability for the delivery of imaging and/or therapeutic payloads. We have engineered constructs derived from erythrocytes, and doped with the FDA-approved near infrared dye, indocyanine green (ICG). We refer to these optical particles as NIR erythrocyte-mimicking transducers (NETs). A particular feature of NETs is that their diameters can be tuned from micron- to nano-scale. Herein, we investigated the effects of micron- (≈2.6 μm diameter), and nano- (≈145 nm diameter) sized NETs on their biodistribution, and evaluated their acute toxicity in healthy Swiss Webster mice. Following tail vein injection of free ICG and NETs, animals were euthanized at various time points up to 48 hours. Fluorescence analysis of blood showed that nearly 11% of the injected amount of nano-sized NETs (nNETs) remained in blood at 48 hours post-injection as compared to ≈5% for micron-sized NETs (μNETs). Similarly, at this time point, higher levels of nNETs were present in various organs including the lungs, liver, and spleen. Histological analyses of various organs, extracted at 24 hours post-injection of NETs, did not show pathological alterations. Serum biochemistry profiles, in general, did not show elevated levels of the various analyzed biomarkers associated with liver and kidney functions. Values of various hematological profiles remained within the normal ranges following the administration of μNETs and nNETs. Results of this study suggest that erythrocyte-derived particles can potentially provide a non-toxic platform for delivery of ICG.


 Please wait while we load your content...
Please wait while we load your content...