Label-free characterization of biochemical changes within human cells under parasite attack using synchrotron based micro-FTIR†
Abstract
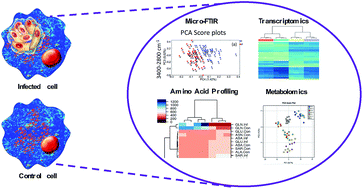
The protozoan Toxoplasma gondii is responsible for severe, potentially life-threatening, infection in immunocompromised individuals and when acquired during pregnancy. In the meantime, there is no available vaccine and the anti-T. gondii drug arsenal is limited. An important challenge to improve antiparasitic therapy is to understand chemical changes that occur during infection. Here, we used Fourier transform infrared spectroscopy (FTIR) to investigate the effect of T. gondii infection on the chemical composition of human brain microvascular endothelial cells (hBMECs) at 3, 6, 24 and 48 hours postinfection (hpi). Principal component analysis (PCA) showed that the best separation and largest difference between infected and uninfected hBMECs was detected at 24 hpi and within the 3400–2800 cm−1 region. At 48 hpi, although the difference between samples was obvious within the 3400–2800 cm−1 region, more differences were detected in the fingerprint region. These findings indicate that infected and control cells can be easily distinguished. Although differences between the spectra varied, the separation was most clear at 24 hpi. T. gondii increased signals for lipids (2853 cm−1) and nucleic acids (976 cm−1, 1097 cm−1 and 1245 cm−1), and decreased signals for proteins (3289 cm−1, 2963 cm−1, 2875 cm−1) in infected cells compared to controls. These results, supported by amino acid levels in culture media, and global metabolomic and gene expression analyses of hBMECs, suggest that T. gondii parasite exploits a wide range of host-derived chemical compounds and signaling pathways for its own survival and proliferation within host cells. Our data demonstrate that FTIR combined with chemometric analysis is a valuable approach to elucidate the temporal, infection-specific, chemical alterations in host cells at a single cell resolution.


 Please wait while we load your content...
Please wait while we load your content...