Carbohydrate isomer resolution via multi-site derivatization cyclic ion mobility-mass spectrometry†
Abstract
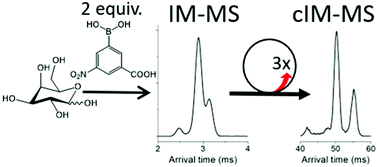
Oligosaccharides serve many roles in extant life and may have had a significant role in prebiotic chemistry on the early Earth. In both these contexts, the structural and isomeric diversity among carbohydrates presents analytical challenges necessitating improved separations. Here, we showcase a chemical derivatization approach, where 3-carboxy-5-nitrophenylboronic acid (3C5NBA) is used to label vicinal hydroxyl groups, amplifying the structural difference between isomers. We explore the applicability of state-of-the-art ion mobility – mass spectrometry (IM-MS) instrumentation in the analysis of derivatized carbohydrates. In particular we focus on the resolving power required for IM separation of derivatized isomers. A recently developed cyclic ion mobility (cIM) mass spectrometer (MS) was chosen for this study as it allows for multi-pass IM separations, with variable resolving power (Rp). Three passes around the cIM (Rp ∼ 120) enabled separation of all possible pairs of four monosaccharide standards, and all but two pairs of eight disaccharide standards. Combining cIM methodology with tandem mass spectrometry (MS/MS) experiments allowed for the major products of each of the 3C5NBA carbohydrate derivatization reactions to be resolved and unequivocally identified.


 Please wait while we load your content...
Please wait while we load your content...
