Near-infrared fluorescent aza-BODIPY dyes for sensing and imaging of pH from the neutral to highly alkaline range†
Abstract
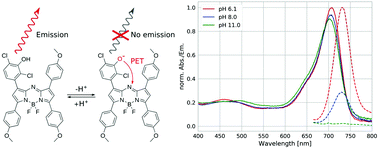
New aza-BODIPY pH indicators with spectral properties modulated solely by photoinduced electron transfer (PET) are presented. The pH sensitive hydroxyl group is located in the meta-position of a phenyl substituent with respect to the aza-BODIPY core, which eliminates the conjugation to the chromophore. The new dyes show reversible “on”–“off” fluorescence response upon deprotonation of the receptor but no changes in the absorption spectrum, which is in contrast to state-of-the-art indicators of the aza-BODIPY family. This eliminates potential changes in the efficiency of the inner filter effect and Förster resonance energy transfer (FRET) and makes the new dyes suitable acceptors in light harvesting systems used for ratiometric pH imaging. The introduction of electron-withdrawing or electron-donating groups into the receptor results in a set of indicators suitable for measurements from physiological (pH 7) to very alkaline (pH 13) conditions. The new sensors are particularly promising for monitoring of pH changes in concrete, as was recently shown elsewhere.
- This article is part of the themed collection: Analyst Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...