Thalidomide-type teratogenicity: structure–activity relationships for congeners
Abstract
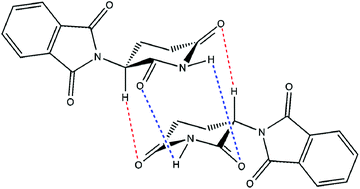
Unravelling the molecular basis of thalidomide embryotoxicity, which is remarkably species–specific, is challenging in view of its low toxicity in the mature animal. Employing data derived solely from proven sensitive primate species or susceptible strains of rabbit, the structure–activity relationship of over 50 compounds which are, arguably, congeners of thalidomide has been reviewed. The molecular requirement for ‘thalidomide-type’ teratogenicity was highly structure dependent. Both the phthalimide and glutarimide groups were essential for embryopathic activity, although minor substitutions in either or both rings could be tolerated without a loss of toxicity. An α-linkage between the two cyclic structures was essential; a β-link resulted in a complete loss of embryopathic activity. Crucially, this α-configuration provided a centre of asymmetry enabling the existence of stereoisomers. The thalidomide molecule is not a static entity and under physiological conditions it undergoes a number of intra- and inter-molecular reactions. Besides irreversible hydrolysis, its keto–enol tautomerism, base-assisted proton transfer and glutarimide ring rotation lead to rapid interconversion of the thalidomide enantiomers. These enantiomers form equilibria between themselves and also between both homochiral and heterochiral dimers. It is proposed that the more energetically favourable and stable heterochiral dimer of thalidomide is an active agent that possesses the structural features of the paired nucleotides of the double-stranded DNA. Its capacity to enter into hydrogen bonding interactions affects DNA expression in a chaotic manner without causing permanent mutations. This disruption may well be concentrated at nucleotide sites known to be involved in specific promoter regions of the genome.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...