Direct observation of adsorption kinetics on clays by cation–π interaction-triggered aggregation luminescence†
Abstract
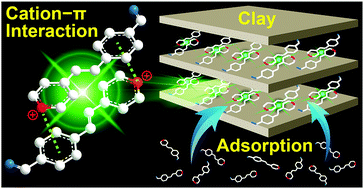
Luminescence quenching of organic molecules in an aggregation state has become a long-standing challenge for further imaging applications. Inspired by recent research on cation–π interaction triggered luminescence, we designed and synthesized an organic cation, E-4-formyl-styryl-pyridinium salt (FSPH), with strong fluorescent emission in the aggregation state. The formation of the FSPH dimer replaces weaker π–π interactions with stronger cation–π interactions to trigger the aggregation luminescence. The excellent optical performances of FSPH in the aggregation state show promise in elucidating the adsorption kinetics of clays because the aggregation of adsorbates during clay adsorption is inevitable. Expectedly, the complete adsorption kinetics of FSPH on clays was visualized by virtue of an in situ fluorescence imaging technique. The subsequent fluorescence intensity quantification revealed that the adsorption kinetics of FPSH on clays could be divided into three stages: molecular aggregation at the edge, formation of a block layer, and molecular invasion from the edge to the center. The discovery of the formation of a block layer not only identifies a previously unknown source of the lower adsorption capacity with larger particle sizes of clays, but also inspires the great passion of scientists to study the adsorption kinetics of other adsorbents by employing cation–π interaction-triggered aggregation luminescence.


 Please wait while we load your content...
Please wait while we load your content...