Substituents engineered deep-red to near-infrared phosphorescence from tris-heteroleptic iridium(iii) complexes for solution processable red-NIR organic light-emitting diodes†
Abstract
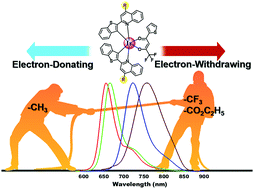
Research on near-infrared- (NIR-) emitting materials and devices has been propelled by fundamental and practical application demands surrounding information-secured devices and night-vision displays to phototherapy and civilian medical diagnostics. However, the development of stable, highly efficient, low-cost NIR-emitting luminophores is still a formidable challenge owing to the vulnerability of the small emissive bandgap toward several nonradiative decay pathways, including the overlapping of ground- and excited-state vibrational energies and high-frequency oscillators. Suitable structural designs are mandatory for producing an intense NIR emission. Herein, we developed a series of deep-red to NIR emissive iridium(III) complexes (Ir1–Ir4) to explore the effects of electron-donating and electron-withdrawing substituents anchored on the quinoline moiety of (benzo[b]thiophen-2-yl)quinoline cyclometalating ligands. These substituents help engineer the emission bandgap systematically from the deep-red to the NIR region while altering the emission efficiencies drastically. Single-crystal X-ray structures authenticated the exact coordination geometry and intermolecular interactions in these new compounds. We also performed an in-depth and comparative photophysical study in the solution, neat powder, doped polymer film, and freeze matrix at 77 K states to investigate the effects of substitution on the excited-state properties. These studies were conducted in conjunction with density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations. Most importantly, the –CH3 substituted Ir1, unsubstituted Ir2, and –CF3 substituted complex (Ir4) were promising novel compounds with bright phosphorescence quantum efficiency in doped polymer films. Using these novel molecules, deep-red to NIR emissive organic light-emitting diodes (OLEDs) were fabricated using a solution-processable method. The unoptimized device exhibited maximum external quantum efficiency (EQE) values of 2.05% and 2.11% for Ir1 and Ir2, respectively.


 Please wait while we load your content...
Please wait while we load your content...