Why are S–F and S–O non-covalent interactions stabilising?†
Abstract
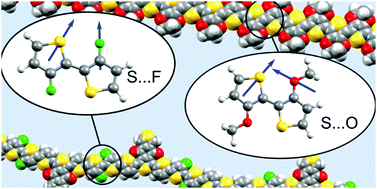
Soft non-covalent interactions are important to bulk property materials, but can also affect molecular or single polymer chain properties in optoelectronic materials. S–F and S–O interactions are often used in material design to planarise π-systems and increase conjugation length, but how these interactions might actually be stabilising is still not fully understood. Here, computational analysis using symmetry adapted perturbation theory and natural bond orbital methods uncovers the key electrostatic interactions between sulfur and neighbouring heteroatoms within the same molecule or polymer chain. A future design rule for materials hoping to affect torsional conformation using these types of interactions is to consider the direction of dipoles of functional groups.
- This article is part of the themed collection: Celebrating our 2020 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...