A2–A1–D–A1–A2 type non-fullerene acceptors based on methoxy substituted benzotriazole with three different end-capped groups for P3HT-based organic solar cells†
Abstract
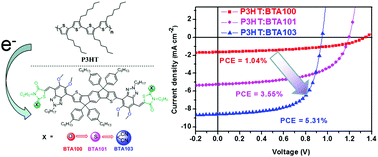
In the last three years, the A2–A1–D–A1–A2 skeleton has become increasingly popular in the design of non-fullerene acceptors (NFAs), and it could match particularly well with the classic p-type polymer of poly(3-hexylthiophene) (P3HT). In this manuscript, we successfully synthesized three NFAs with this skeleton, named BTA100, BTA101 and BTA103, where BTA units were substituted by methoxy groups and used as the middle electron-accepting unit (A1). To fine-tune the energy levels of the final BTA-based NFAs, three different electron-deficient building blocks, thiazolidine-2,4-dione (TD), rhodanine (R) and 2-(1,1-dicyanomethylene)rhodanine (RCN), were used as the end groups (A2), respectively. The introduction of methoxy groups into BTA can upshift the lowest unoccupied molecular orbital (LUMO) energy level of NFAs and realize high open-circuit voltage (VOC) organic solar cells. In addition, the O atom shows a weak interaction with the S atom in the neighbouring thiophene ring, which might be able to facilitate intramolecular charge transfer. The organic solar cell (OSC) device based on P3HT:BTA103 shows a high PCE of 5.31% with a VOC of 0.94 V, a JSC of 8.56 mA cm−2 and a FF of 0.66. In addition, it is worth noting that the VOC of P3HT:BTA100 reached 1.34 V, which is one of the highest values for P3HT based solar cells. These results indicate that RCN is also an effective end group to construct NFAs and methoxy substitution is a simple method to improve the VOC for P3HT-based OSC devices.


 Please wait while we load your content...
Please wait while we load your content...