Butterfly-shaped asymmetric squaraine dimers for organic photovoltaics†
Abstract
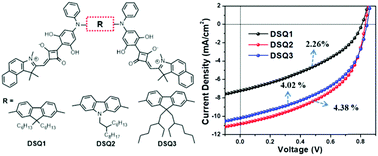
Squaraines have been demonstrated as one class of effective low-bandgap donor materials in bulk-heterojunction organic solar cells (BHJ-OSCs). However, they often suffer from low hole mobility, thereby limiting their photovoltaic performance. Herein, we designed and synthesized three new butterfly-shaped dimeric squaraines (DSQ1–3) with an extended π-conjugated system for BHJ-OSCs. Our results reveal that the modification of connecting bridges between two asymmetric dyes has a minimal effect on their absorption, energy levels and charge transport properties, however, subtle side chain modifications can efficiently improve the charge transport ability in the BHJ films due to enhanced miscibility with fullerene acceptors. By simply replacing the hexyl chains in the fluorene core of DSQ1 by branched 2-ethylhexyl chains, the resulting DSQ3 could possess a hole mobility of 1.10 × 10−4 cm2 V−1 s−1, providing an ∼6 times enhancement of that of DSQ1. Thus, as donor materials in single-junction BHJ-OSCs, DSQ3 afforded a power conversion efficiency (PCE) of 4.02%, while that of DSQ1 was only 2.26%. Moreover, the 2-hexyldecyl substituted carbazole cored DSQ2 showed slightly higher hole mobility than DSQ3, and thus generated the highest PCE of 4.38% among the three dimers. Our work strengthens the importance of side chain engineering for designing high-performance squaraine-based donor materials, even for those already having a large π-conjugated system.


 Please wait while we load your content...
Please wait while we load your content...