Photoluminescence in m-carborane–anthracene triads: a combined experimental and computational study†
Abstract
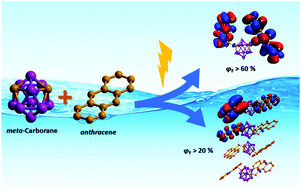
New hybrids synthesized by linking two anthracenyl units to the Ccluster atoms of a non- (4), a mono- (5) and a di-iodinated (6) m-carborane fragment through CH2 spacers, along with their full characterization, are reported. Noticeably, bonding the m-carborane fragment to the anthracene moieties produces a significant increase of more than two-fold in the intrinsic fluorescence quantum yield (ϕF) of the anthracene itself, with values of ϕF > 60% in THF and ϕF > 48% in toluene, although it does not alter the absorption and emission patterns of the fluorophore in solution. A red-shift of the emission maximum with respect to the solution is observed in the aggregate state (THF/H2O, 1 : 99 v/v), along with moderate quantum yields; compounds 4 and 5 show ϕF = 22 and 19%, respectively, whereas 6 has a lower value (ϕF = 8%). The difference between the ϕF values in the aggregate state has been attributed to the arrangement of dimers for each compound in the solid state structures. X-ray crystal structures of compounds 4 and 5 show the anthracene units to be roughly parallel, whereas such an arrangement is clearly disrupted in compound 6. Such differences have been analyzed by Hirshfeld surfaces, decomposed fingerprint plots for the three compounds and DFT calculations. The combined results from the supramolecular analyses and DFT studies support the idea that a less delocalized system in the case of 6 can be explained by the different packing in the aggregate or solid state for this di-iodo derivative. The observed arrangement of molecules of 6 seems to be related to a larger number of H⋯I contacts, with respect to the non-iodinated or mono-iodinated compounds, 4 or 5. According to this assumption, there is a direct relationship between the structure in the solid state and the PL properties; in the m-carborane derivatives, small changes in their structures have caused variations in the photophysical properties, especially in the quantum efficiency.


 Please wait while we load your content...
Please wait while we load your content...