ortho-Fluorination of azophenols increases the mesophase stability of photoresponsive hydrogen-bonded liquid crystals†‡
Abstract
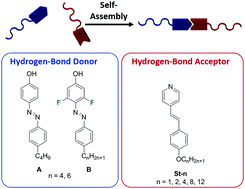
Photoresponsive liquid crystals (LCs) whose alignment can be controlled with UV-Visible light are appealing for a range of photonic applications. From the perspective of exploring the interplay between the light response and the self-assembly of the molecular components, supramolecular liquid crystals are of particular interest. They allow elaborating the structure–property relationships that govern the optical performance of LC materials by subtle variation of the chemical structures of the building blocks. Herein we present a supramolecular system comprising azophenols and stilbazoles as hydrogen-bond donors and acceptors, respectively, and show that ortho-fluorination of the azophenol dramatically increases the thermal stability of the LC phases, an important characteristics in their further utilization in photonics. The systems exhibit fast photoinduced order–disorder transitions, and rapid recovery of the liquid-crystalline state once the light irradiation is ceased, due to the photochemical properties of azophenols.
- This article is part of the themed collection: Photonics


 Please wait while we load your content...
Please wait while we load your content...