Effects of morphology of Mg powder precursor on phase formation and superconducting properties of Mg11B2 low activation superconductor†
Abstract
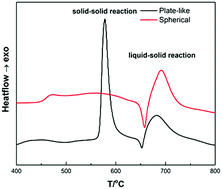
The effect of morphology with magnesium (Mg) powder on phase formation and critical current density (Jc,) of sintered Mg11B2 bulk was studied. As a precursor for the formation of Mg11B2, the morphology of spherical and plate-like of Mg powder were separately synthesized using atomization and chemical reduction methods. From differential thermal analyses of the sintering process, the solid–solid reaction of a Mg11B2 sample using spherical Mg powder was confirmed to be much weaker than the plate-like one, and the second exothermic peak place was shifted back and its intensity was increased due to a special microstructure of spherical Mg powder. XRD analysis of Mg11B2 samples showed that the volume fraction of Mg11B2 in the sintered sample with plate-like Mg was higher than that with the spherical one, and for all the samples MgB2 is the main phase. The Mg11B2 sample using spherical Mg powder had two different kinds of microstructures because of differences in their diffusivity and their contact interfaces. The Jc value of an Mg11B2 sample using spherical Mg powder enhanced the entire field; it was twice as high as that using plate-like Mg, and it was even better than all the Jc values reported in previous literature using the same kind of Mg powder as precursor.


 Please wait while we load your content...
Please wait while we load your content...