Solvent-vapor-annealed A–D–A-type semicrystalline conjugated small molecules for flexible ambipolar field-effect transistors†
Abstract
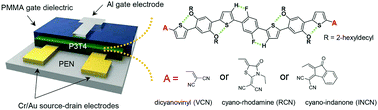
This paper reports a series of acceptor–donor–acceptor (A–D–A)-type small molecules (named P3T4-VCN, P3T4-RCN, and P3T4-INCN) based on an oligothiophene–phenylene core with three different electron-accepting terminal groups—dicyanovinyl (VCN), cyano-rhodanine (RCN), and cyano-indanone (INCN), respectively—for application to flexible ambipolar organic field-effect transistors (OFETs). Intrachain noncovalent coulombic interactions (via S–F and H–F interactions) were incorporated into the design of the P3T4 backbone to enhance the chain planarity. All the P3T4-based OFETs exhibited ambipolar behavior with hole-dominant transport, and the OFET performances were strongly dependent on the terminal groups. The P3T4-INCN OFET exhibited the highest carrier mobility owing to the extended π-conjugation via the INCN moiety, which enhanced the intermolecular cofacial π–π stacking and generated an efficient carrier pathway in the transistor channel. Room temperature solvent vapor annealing resulted in a dramatic increase in the carrier mobility of the OFETs without causing any damage to a polyethylene naphthalate (PEN) plastic substrate. The effects of both the terminal groups of the P3T4 small molecules and solvent vapor annealing were systematically investigated by UV-vis absorption spectroscopy, two-dimensional grazing incidence X-ray diffraction, and atomic force microscopy. In addition, a flexible OFET array with solvent-vapor-annealed P3T4-INCN was successfully fabricated on a PEN substrate. These OFET devices exhibited a hole mobility of 0.15 cm2 V−1 s−1, an electron mobility of 0.05 cm2 V−1 s−1, an on–off current ratio of ∼105, and excellent mechanical stability even after 300 bending cycles.


 Please wait while we load your content...
Please wait while we load your content...