A simple and broadly applicable synthesis of fluorene-coupled D–σ–A type molecules: towards high-triplet-energy bipolar hosts for efficient blue thermally-activated delayed fluorescence†
Abstract
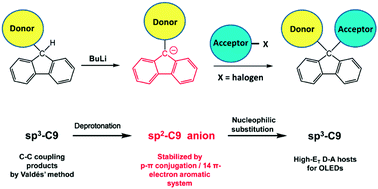
An effective two-step synthesis of high-triplet-energy (ET) bipolar hosts that is simple and broadly applicable is introduced. Electron-donating (D = (9-phenyl-9H-carbazol-3-yl/4-(9H-carbazol-9-yl)phenyl)) and -accepting (A = (tetrafluoropyridin-4-yl/4,6-diphenyl-1,3,5-triazin-2-yl)) units have been coupled to the C9 atom of fluorene to afford the four hosts 3CzFTFP, 9PhFTFP, 3CzFDPhTz, and 9PhFDPhTz with sp3 C9-centered bulky ternary structures, excellent thermal/morphological stabilities, tunable abilities for bipolar charge carrier injection/transport, and identical high ET (2.87 eV). Blue thermally-activated delayed fluorescence (TADF) devices using these new hosts and 2CzPN as the host–guest system exhibited efficient electroluminescence efficiencies of 38 cd A−1/30 lm W−1/17%, which are among the best for 2CzPN-based TADF devices. The generality of this synthetic strategy to high-ET fluorene-coupled D–σ–A type hosts was confirmed by successfully appending other D/A groups ((diphenylamino)phenyl/phenylsulfonyl) to fluorene, affording TPAFBSO.


 Please wait while we load your content...
Please wait while we load your content...