Impact of the use of sterically congested Ir(iii) complexes on the performance of light-emitting electrochemical cells†‡
Abstract
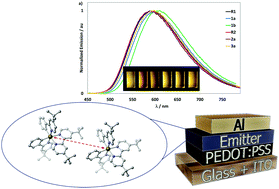
The synthesis, structural and optoelectronic characterization of a family of sterically congested cyclometalated cationic Ir(III) complexes of the form [Ir(C^N)2(dtBubpy)]PF6 (with dtBubpy = 4,4′-di-tert-butyl-2,2′-bipyridine and C^N = a cyclometalating ligand decorated at the 4-position of the pyridine ring and/or the 3-position of the phenyl ring with a range of sterically bulky substituents) are reported. This family of complexes is compared to the unsubstituted analogue complex R1 bearing 2-phenylpyridinato as cyclometalating ligand. The impact of sterically bulky substituents on the C^N ligands on both the solid state photophysics and light-emitting electrochemical cell (LEEC) device performance is investigated. X-ray diffraction analysis of complexes 1a, R2, 2a, and 1b show an increasing internuclear distance in the solid state, within these four complexes. Emission studies in solution and neat film show that the chosen substituents essentially do not impact the emission energy. The photoluminescence quantum yields (ΦPL) are in the same range (ΦPL ∼ 25–31%), except for 1b, which shows a lower ΦPL of 12%. All complexes exhibit similar monoexponential emission lifetimes in the submicrosecond regime. LEECs based on R1, 1a, 1b and R2 were fabricated, showing yellow luminescence and moderate efficiencies and lifetimes. The arguably best performing LEEC device, showing the highest luminance (737 cd m−2), current efficiency (7.4 cd A−1) and EQE (2.6%), employed emitter 1a.


 Please wait while we load your content...
Please wait while we load your content...