Liquid-ammonia synthesis of microporous Mg3N2 showing intense red-light emission†
Abstract
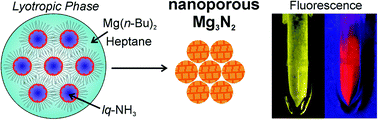
Magnesium nitride (Mg3N2) is prepared via lyotropic phases with liquid ammonia (lq-NH3). To avoid oxide contamination, the synthesis was performed in the absence of any oxygen source (e.g., solvents, starting materials, and surfactants). Specifically, the lyotropic phase was established by lq-NH3 at −50 °C as the polar phase, heptane as the non-polar dispersant phase, dimethyldioctylammonium iodide (DDAI) as the surfactant, hexylamine as the cosurfactant, and di-n-butylmagnesium as the starting material. Ammonolysis was completed by slow heating to 500–600 °C in a nitrogen atmosphere (N2). Structure and purity – especially the absence of oxygen – were studied in detail with different electron microscopic and electron spectroscopic techniques. The as-obtained yellow Mg3N2 (heated at 500 °C) exhibits high porosity (specific surface area: 176 m2 g−1; total pore volume: 0.41 cm3 g−1; and micropore volume: 0.13 cm3 g−1) and good selectivity regarding CO2 adsorption (CO2 uptake: 108 mg g−1; N2 uptake: 9 mg g−1; both at 90 bars). After heating to 600 °C, the as-obtained yellow Mg3N2 (band gap: 2.89 eV) shows intense red emission (600–900 nm) with a quantum yield of 29 ± 3%. Liquid-phase synthesis, oxygen-free Mg3N2 and intense red emission are reported for the first time, including nanostructured as well as bulk Mg3N2.


 Please wait while we load your content...
Please wait while we load your content...