Carboxyl-functionalized ionic liquids: synthesis, characterization and synergy with rare-earth ions†
Abstract
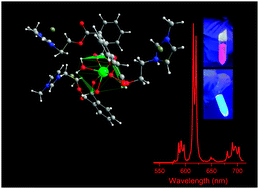
Herein, we describe for the first time room temperature ionic liquids (RTILs) and imidazole-based cations with appended carboxylic acids as terminals, which are directly derived from the anhydrides. All structures were designed to meet the following criteria: (1) easy preparation (up-scaling into kg scale possible); (2) melting point of <25 °C, aligned with a thermal stability of up to 100 °C; (3) chemical stability in water and common organic solvents; (4) relatively high viscosity; and (5) featured luminescence. One of the central questions in our work is how ligands can enforce a specific structure and how this enforcement may lead to several properties. Luminescent, flexible, stable, and transparent ionogels were obtained from ILs in coordination with lanthanide ions (Eu or Gd). Ionogels combine the properties of ionic liquids and show outstanding luminescence properties with decay times >1 ms, high quantum efficiency (47%), featured quantum yield (27%), and a remarkable sensitization efficiency to ILs of up to 83%, which suggests a synergistic coordination with lanthanide ions. We show that the materials obtained are potentially applicable for the construction of light-emitting electrochemical cells, owing to the properties of their basic components, including ionic liquids (with high electrical conductivity and a similar structure to liquid crystals) and lanthanide ions (which have unprecedented photophysical properties).
- This article is part of the themed collections: International Year of the Periodic Table : Lanthanides for Precision Therapy and Beyond and Materials and Nano Research in Brazil


 Please wait while we load your content...
Please wait while we load your content...