Tuning the optoelectronic characteristics of ionic organic crystalline assemblies†
Abstract
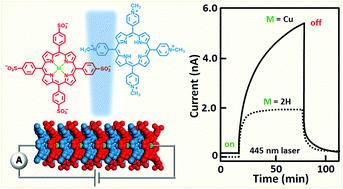
The effect of selective metallation of free-base ionic porphyrin tectons on the structural, electronic, and optical properties of their crystalline self-assemblies is presented. Rod-like crystals were prepared under neutral pH conditions by combining meso-tetra(N-methyl-pyridyl)porphyrin, H2TMPyP, and meso-tetra(4-sulfonatophenyl)porphyrin, H2TSPP, with either a nickel or a copper ion contained in one of the synthons. These materials were characterized by optical microscopy, X-ray diffraction methods, thermogravimetric analysis, diffuse reflectance UV-visible and luminescence spectroscopies, and conductivity and photoconductivity measurements. All the porphyrin assemblies formed monoclinic P21/c crystals with pseudo-hexagonal cross-sections. Thermogravimetric experiments indicate that water molecules associated with crystals desorb at two different rates. In addition, temperature dependent XRD showed that the dehydration of the porphyrin solids causes modification in the crystals which is completely reversible up to 100 °C annealing (i.e., crystals return to their original structural geometry upon rehydration). All the metallated porphyrin crystals exhibit dark conductivity at moderately high temperatures and become more conductive upon photoexcitation. The photoresponse of the H2TMPyP:CuTSPP-substituted crystals is significantly higher than that of the CuTMPyP:H2TSPP and the Ni-substituted crystals. The Cu-substituted systems, like the parent free-base, exhibit persistent photoconductivity resulting from excitations into the Q and Soret bands. The primary charge carriers in these solids upon photoexcitation are electrons and the charge recombination mechanism follows monomolecular kinetics. Quantum mechanical calculations provide the electronic band structure and the density of states and explain the experimental prompt photoconductivity measurements of the porphyrin self-assemblies. This work provides evidence that optoelectronic properties of organic semiconductors can be effectively tuned by introducing transition metals into their crystal structures.


 Please wait while we load your content...
Please wait while we load your content...