Interplay between single-ion magnetism, single-chain magnetism and long-range ordering in the spin chain oxides Sr4−xCaxMn2CoO9
Abstract
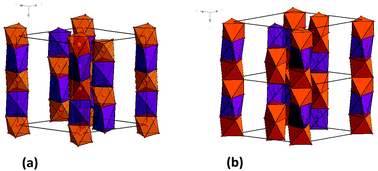
Triangular spin chain oxides of the formula Sr4−xCaxMn2CoO9 have been synthesized for a large compositional range, 0 ≤ x ≤ 2.7, allowing the study of the impact of chemical pressure on their magnetic properties. Six compositions were investigated in detail by dc magnetization, ac susceptibility and specific heat measurements. These oxides exhibit two types of magnetic responses which are generally regarded as being typical of molecular materials: Single-Ion Magnet (SIM) and Single-Chain Magnet (SCM). For x = 0, coexistence of SIM and SCM behaviors is observed. In the range 0 < x ≤ 1, some features reveal the appearance of a short-range ordering in a temperature range slightly above that of the SIM/SCM dynamic responses. For x ≥ 1.5, the chemical pressure becomes large enough to induce a magnetic Long-Range Ordering (LRO). In this x range, the transition temperature significantly increases with x, from 22 K for x = 1.5 up to 35 K for x = 2.7. The SIM behavior is found to persist over the whole composition range, coexisting either with SCM (x ≤ 1) or LRO (x ≥ 1.5). These results are discussed with regard to those previously reported in molecular compounds about the interplay between SIM, SCM and LRO.


 Please wait while we load your content...
Please wait while we load your content...