Blue versus yellow emission in bipolar fluorenone derivatives: the impact of aggregation and hydrogen bonding†
Abstract
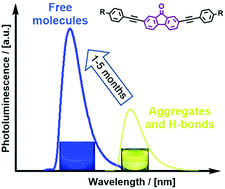
Three donor–π−acceptor–π-donor multichromophore fluorenone-centered compounds are presented. The π-bridges between the fluorenone and carbazole donors contain ethynyl moieties linked to various branched spacing moieties, such as triphenylamine, 1,3,5-triphenylbenzene and 9-phenylcarbazole. Differences in the photophysical, photoelectrical, thermal and charge-transporting characteristics of the three compounds are analyzed by means of experimental and density functional theory (DFT) methods. The investigated compounds exhibit good ambipolar charge transport behavior with mobility values exceeding 10−3 cm2 V−1 s−1. The compounds exhibit interesting photoluminescence properties, which are strongly influenced by the important tendency of these compounds in solution to form strongly bound ground state aggregates. Photoluminescence emission of these materials is found to originate predominantly from intramolecular transitions of π–πCT* nature which experience a strong redshift and an intensity decrease from the aggregation and intermolecular H-bonds. A slowly evolving equilibrium between strongly aggregated molecules and free ones is observed in THF solutions, resulting in the emission color swapping gradually from yellow to blue with a drastic intensity increase. The correlation of the aggregation strength with the absorption and emission properties, photoluminescence quantum yield, and the time needed for color swapping is discussed. Furthermore, seldom observed photoluminescence intensity enhancement with temperature increase was detected in non-polar media.


 Please wait while we load your content...
Please wait while we load your content...