Facile synthesis of magnetic fluorescent nanoparticles: adsorption and selective detection of Hg(ii) in water†
Abstract
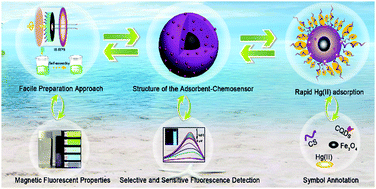
An adsorbent–chemosensor system based on magnetic fluorescent nanoparticles (MFNPs) was successfully constructed via high-gravity technology and a self-assembly approach, which adopts magnetic Fe3O4 nanoparticles as a core, chitosan (CS) as a shell, and carbon quantum dots (CQDs) as a fluorescent probe. The adsorbent–chemosensor possesses an average diameter of 10 nm and a narrow size distribution, as well as superparamagnetic properties, a well-defined pore structure, and superior fluorescence emission intensity. As an adsorbent, the core–shell nanocomposites provide a relatively high adsorption capacity for heavy metal ions and displayed a maximum monolayer adsorption capacity of 110.62 mg Hg(II) per gram of adsorbent owing to the synergistic interaction due to the high porosity of the core–shell structure and the high affinity of Hg(II)–Fe3O4 or Hg(II)–CS interactions. As a fluorescent probe, the as-prepared hybrid nanospheres exhibited favorable sensitivity and a low detection limit (12.43 nM) toward Hg(II). The nanocomposites also demonstrated excellent reusability, with a loss of adsorption of less than 13% and a loss of fluorescence intensity of 5% after five regeneration test cycles. The present work provides a potential approach for the design of a novel and cheap adsorbent–chemosensor for application in the recognition of Hg(II) and its elimination from water.


 Please wait while we load your content...
Please wait while we load your content...