4,5-Diazafluorene co-oligomers as electron-deficient light-emitting materials and selective fluorescence sensors for mercury(ii) cations†‡
Abstract
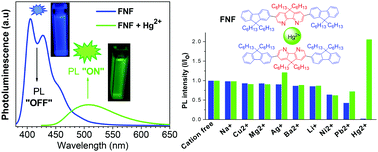
A series of 4,5-diazafluorene-based (N) conjugated co-oligomers with 9,9-dialkylfluorene (F) or electron-deficient dibenzothiophene-S,S-dioxide (S) has been synthesized by Pd-catalyzed coupling reaction (FNF, FFNFF, FNoF, SNS, NSN). Cyclic voltammetry studies reveal their improved electron affinity compared to oligofluorene. SNS and NSN co-oligomers showed a decrease of their LUMO energies by 0.37–0.38 eV compared to FNF co-oligomer. Absorption/emission studies showed that all oligomers, except FNoF, are blue-emitting materials (λPL ∼ 400–450 nm) with high quantum yields of their photoluminescence (ΦPL = 84–100% in solution and 24–42% in the solid state). FNoF trimer emits in the yellow region with a very low ΦPL ∼ 1% in dichloromethane solution, but the emission efficiency is substantially increased to ΦPL = 10–17% in the solid state. FNF co-oligomer was studied as a metal cation responsive colorimetric and fluorescent sensor using a series of mono- and divalent cations and it showed high sensitivity and selectivity toward mercury cations. Upon addition of Hg2+, the blue emission of FNF (λPL = 404 nm) was quenched and a new, bathochromically shifted (to the green region, λPL = 507 nm) emission band appeared, which allows this compound to be used as both an “ON → OFF” and “OFF → ON” fluorescent sensor.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...