Inversion of charge carrier polarity and boosting the mobility of organic semiconducting polymers based on benzobisthiadiazole derivatives by fluorination†
Abstract
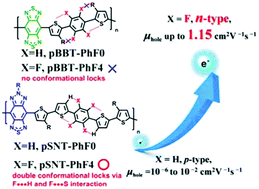
One of the most effective molecular design strategies in organic electronics is fluorination. In our research program of benzobisthiadiazole (BBT)-based semiconducting polymers, this strategy was employed for the first time to obtain fluorinated (F)-polymers, namely, pBBT-PhF4 and pSNT-PhF4. The fluorination effects on the optical, electrochemical, molecular assembling and charge-transport properties of these polymers were comprehensively studied. While the fluorination hardly affected the optical band gap, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels significantly decreased, leading to the inversion of charge carrier polarity from p-type to n-type in the case of pBBT-PhF4. Further studies of the thin film microstructures, evaluated by their grazing-incidence wide-angle X-ray scattering (GIWAXS) patterns and atomic force microscopy (AFM) images, demonstrated that pSNT-PhF4 adopted an edge-on orientation, while its non-F-counterpart adopted a face-on orientation. Due to its very high crystallinity, shorter π–π stacking distance and edge-on backbone-orientation, pSNT-PhF4 showed a hole mobility as high as 1.15 cm2 V−1 s−1. This simple and effective design strategy will guide the way to developing versatile semiconducting polymers for transistor applications with a tunable charge carrier polarity.
- This article is part of the themed collection: Celebrating 50 years of Professor Fred Wudl’s contributions to the field of organic semiconductors


 Please wait while we load your content...
Please wait while we load your content...