Synthesis and remarkable mechano- and thermo-hypsochromic luminescence of a new type of DPP-based derivative†
Abstract
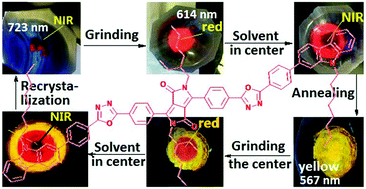
Owing to academic significance and potential applications, mechanofluorochromism has attracted much research interest. However, among the hundreds of mechanofluorochromic organic and metallorganic compounds reported to date, those with high contrast, multicolour and multichannel switching, and a remarkable mechanohypsochromic luminescence are rarely realized. This paper presents the synthesis and unusual mechano- and thermo-chromic fluorescence behaviours of a new 1,4-diketo-pyrrolo[3,4-c]pyrrole derivative (CODPP) with oxadiazole as the conjugation bridge and carbazole as the end donor. The as-prepared crystalline CODPP emits a near-infrared light (723 nm, NIR state) and can be mechanically ground into a red emitting state (614 nm, R state), rendering a large mechanofluorohypsochromic shift up to 110 nm. However, unlike most mechanofluorochromic materials, the ground R state could not revert to the pristine (NIR) state upon heat annealing; instead, a new yellow emitting state (557 nm, Y state) is formed, and grinding the Y state affords the R state. Moreover, both the Y and R states hardly change their colours upon solvent fuming but can form the NIR state only by recrystallization or reprecipitation. These changes are repeatable and are characterized and discussed based on IR, X-ray diffraction, DSC, and time-resolved fluorescence spectra. This remarkable mechano- and thermo-hypsochromic luminescence has enriched the types of stimuli responsive materials and enlarged the optical properties of DPP derivatives.


 Please wait while we load your content...
Please wait while we load your content...