Microfluidic channels with renewable and switchable biological functionalities based on host–guest interactions†
Abstract
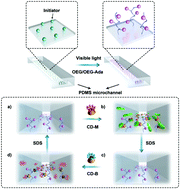
Poly(dimethylsiloxane) (PDMS)-based microfluidic systems are gaining increasing attention due to their ease of fabrication, optical transparency and mechanical properties. However, the inherent hydrophobicity and chemical inertness of PDMS hinder its wider application in microfluidic systems. There is thus a strong need for methods for surface modification of PDMS-based microfluidic channels. In this work, oligo(ethylene glycol)methacrylate (OEGMA) and adamantane-containing OEGMA (OEGMA-Ada) were graft copolymerized on PDMS microchannel surfaces using a simple photochemical process to give PDMS-POA. OEGMA was chosen for its resistance to non-specific protein adsorption, and OEGMA-Ada was chosen for its subsequent attachment of mannose with bacteria binding affinity or biotin with avidin binding affinity. β-CD decorated with biotin (CD-B) and/or mannose (CD-M) was attached to the PDMS-POA microchannels via host–guest interactions between the adamantane and β-CD moieties. The data obtained suggest that the functions of the PDMS-POA/CD-B and PDMS-POA/CD-M microchannels with respect to biotin binding and bacterial adhesion were renewable. In addition, the biofunction of the PDMS-POA microchannels could be switched by treatment with SDS to release the CD component followed by treatment with a different β-CD derivative. Different from previous surface modification strategies for PDMS-based microfluidic channels, the combination of visible light-induced grafting and host–guest chemistry provides modified PDMS microchannels with renewable and switchable biofunctions for the detection and measurement of specific proteins and bacteria.


 Please wait while we load your content...
Please wait while we load your content...