Using single molecule force spectroscopy to facilitate a rational design of Ca2+-responsive β-roll peptide-based hydrogels
Abstract
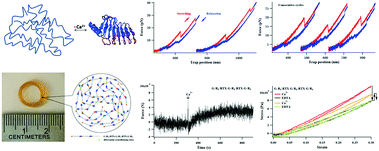
This study demonstrated that incorporation of Ca2+-responsive β-roll peptides arising from repeat-in-toxin (RTX) into elastomeric proteins provided an approach to construct hydrogels that exhibit Ca2+-responsive mechanical properties through a force analysis-based approach. Use of circular dichroism spectroscopy confirmed that there was a Ca2+-induced conformational change of RTX-based recombinant polyproteins. The polyproteins could be crosslinked into solid hydrogels. Shrinking/swelling measurements showed a Ca2+-responsive dimensional change of the RTX-based hydrogels. Mechanical measurements at constant pulling speed and at constant extension suggested that the mechanical properties of the RTX-based hydrogels were Ca2+-responsive. Experimental single molecule force spectroscopies were used to investigate the nano-mechanical stability of the RTX domains. Single molecule atomic force microscopy and optical tweezers provided evidence that the Ca2+-dependent refolding of the intrinsically disordered RTX led to the force increase. The results indicated that the unique Ca2+-responsive mechanical properties of the RTX-based hydrogels at the macroscopic level could be attributed to the nano-mechanical properties of the RTX domains engineered into individual polyproteins at the single molecule level.


 Please wait while we load your content...
Please wait while we load your content...