Functional aza-boron dipyrromethenes for subcellular imaging and organelle-specific photodynamic therapy†
Abstract
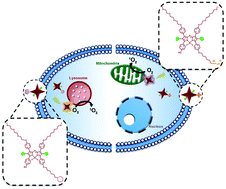
Two hydrophilic aza-boron dipyrromethene (aza-BODIPY) derivatives have first been designed and synthesised for subcellular imaging. With a triphenylphosphonium or morpholine substituent, these probes show high affinity towards the mitochondria or lysosomes of a range of cancer cell lines, including human cervical cancer HeLa cells, hepatocellular carcinoma HepG2 cells, breast cancer MCF-7 cells and colon adenocarcinoma HT29 cells as revealed by confocal microscopy. By introducing two bromo groups into the aza-BODIPY skeleton, which can promote intersystem crossing by the heavy-atom effect, the resulting compounds become efficient singlet-oxygen generators and can serve as organelle-specific photosensitisers for photodynamic therapy (PDT). Upon irradiation, both compounds are photocytotoxic. The lysosome-targeted derivative exhibits higher cellular uptake and more efficient reactive oxygen species generation inside HeLa cells, resulting in higher PDT efficacy with an IC50 value of 0.48 μM and cell death mainly through apoptosis.


 Please wait while we load your content...
Please wait while we load your content...