A simple design of fluorescent probes for indirect detection of β-lactamase based on AIE and ESIPT processes†
Abstract
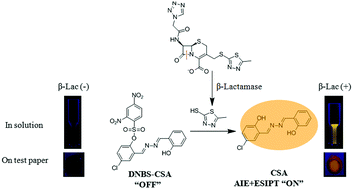
A novel fluorescent probe DNBS-CSA is developed for light-up detection of β-lactamase. The probe design is based on an indirect detection approach with three step reactions. β-Lactamase can react with the lactam of its substrate (cefazolin sodium) to produce a secondary amine, initiating a spontaneous elimination reaction and affording a thiol compound. The thiol could further react with the sulfonate group of DNBS-CSA, releasing the salicylaldehyde azine derivative (CSA) with both aggregation induced emission (AIE) and excited-state intramolecular proton transfer (ESIPT) characteristics. Previously reported β-lactamase probes require covalent linkage of the substrate β-lactam ring part to the probe, which makes probe synthesis difficult due to the complicated structure of the β-lactam ring. In contrast, modification of the β-lactam ring is no longer necessary for DNBS-CSA according to our indirect detection approach. The linear range of fluorescence quantification for β-lactamase is 0–10 mU mL−1 in an aqueous solution. Moreover, owing to the AIE properties of CSA, detection of β-lactamase with DNBS-CSA on test papers was also achieved.


 Please wait while we load your content...
Please wait while we load your content...