Improved cell membrane bioaffinity sample pretreatment technique with enhanced stability for screening of potential allergenic components from traditional Chinese medicine injections
Abstract
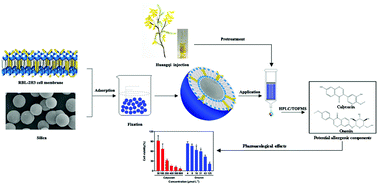
Aiming at improving reliability and tedious analysis time in conventional cell membrane chromatography, an improved bioaffinity sample pretreatment technique with enhanced stability was developed to fast screen and extract potential allergenic components from traditional Chinese medicine injections. In this study, rat basophilic leukemia-2H3 cell membrane coated silica particles (RBL-2H3/CMCSPs) were fabricated by irreversible adsorption between the cell membrane and silica and self-fusion of the cell membrane, which could simulate drug–receptor interactions in vitro. Also, benefiting from the use of paraformaldehyde, the average recoveries of the six batches of RBL/CMCSPs were 90.2% with a relative standard deviation of less than 7.8%, showing that the stability of the materials was remarkably improved compared to materials without fixation. After the successful characterization by spectroscopic and imaging instruments, the prepared RBL-2H3/CMCSPs exhibited desirable adsorption capacity and selectivity. The RBL-2H3/CMCSPs combined with high performance liquid chromatography coupled with time of flight mass spectrometry were then successfully applied to screen and identify two potential allergenic components from huangqi injection, the allergenic activities of which were further investigated by β-hexosaminidase release assay and histamine release assay in vitro. Overall, this work provides a good platform for the fabrication of bioaffinity sample pretreatment materials with high stability and a long lifespan, which can be a time-saving and energy-saving bioaffinity method to rapidly screen and preconcentrate target compounds from complex samples.


 Please wait while we load your content...
Please wait while we load your content...