High throughput identification of Li ion diffusion pathways in typical solid state electrolytes and electrode materials by BV-Ewald method†
Abstract
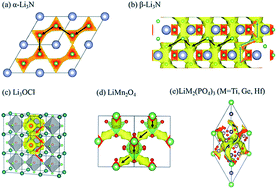
Viewing the possible Li-ion migration pathways in solid state electrolytes and electrode materials is an important prerequisite for the development of all-solid-state battery materials. For high throughput material screening, it is important to have fast methods to determine the possible Li-ion pathways. In this study, by combining the traditional bond valence (BV) method with the Ewald summation method, a new BV-Ewald method was developed to calculate the Li ion diffusion map. The advantage of the new method is the improvement on the traditional BV method by taking into account the cation–cation Coulomb repulsion. By adjusting the isosurface value, the BV-Ewald method can effectively reveal the Li pathways of several well-studied electrolytes (e.g. Li3OCl, LiTi2(PO4)3, α-Li3N and β-Li3N) and cathode materials (e.g. LiFePO4, LiMn2O4) and match with the experimental and ab initio calculation results. Furthermore, this new high throughput method was used for the fast prediction of Li-ion 1D, 2D and 3D-pathways of (anti)perovskite, NASICON, LISICON, garnet, Li-nitride, Li-hydride, Li-halide and argyrodite types of crystals with 34 different known electrodes and electrolytes as representatives. Hence, the new BV-Ewald method will help in the fast discovery of potential solid electrolyte materials.


 Please wait while we load your content...
Please wait while we load your content...
