Desensitization of the dinitromethyl group: molecular/crystalline factors that affect the sensitivities of energetic materials†
Abstract
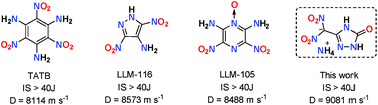
The dinitromethyl group has attracted considerable attention because of its high nitrogen and oxygen content, positive oxygen balance and high energy. Although most dinitromethyl-functionalized compounds suffer from sensitivity to mechanical stimuli, a series of 3-dinitromethanide-1,2,4-triazolone-based energetic salts have now been synthesized and fully characterized. Of these, the ammonium salt is insensitive to impact and friction. X-ray single crystal analysis shows that the ammonium moiety arranges through a close layer-by-layer assembly, which is helpful in decreasing sensitivity. In addition, intramolecular hydrogen-bonding interactions, and intense π–π interactions also are stabilizing factors which impact the sensitivity of the ammonium salt. The enthalpies of formation and energetic properties of the series of salts were determined in order to reach the conclusion that these multiple stabilization factors are effective tools in decreasing the sensitivity of dinitromethyl-based energetic materials.


 Please wait while we load your content...
Please wait while we load your content...
