Ionic liquid containing electron-rich, porous polyphosphazene nanoreactors catalyze the transformation of CO2 to carbonates†
Abstract
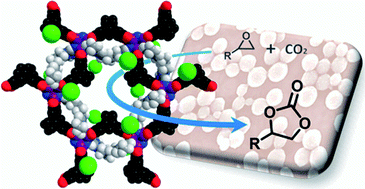
We show that ionic liquids (ILs) interact with electron-rich, porous polyphosphazene (PPZ), to form hybrid PPZ-IL nanoreactors able to simultaneously capture and transform CO2 into carbonates. The PPZ nanospheres swell in organic solvents and effectively absorb IL cations by virtue of the electron-rich sites, while leaving the anions exposed and increasing their nucleophilicity. This leads to considerably higher catalytic activity compared to the IL alone in the cycloaddition reaction of CO2 to epoxides. The cation shielding effect is dependent on the structure of the IL cation and, hence, the catalytic activity can be tuned by varying the structure of the cation in the IL and DFT calculations were used to rationalize the experimentally observed differences in catalytic activity. These studies indicate that PPZ nanospheres could find widespread uses in catalysis, acting as active nanosupports for homogeneous catalysts, not only for the transformations of CO2, but also for other substrates.


 Please wait while we load your content...
Please wait while we load your content...