Selective CO2 electroreduction over an oxide-derived gallium catalyst†
Abstract
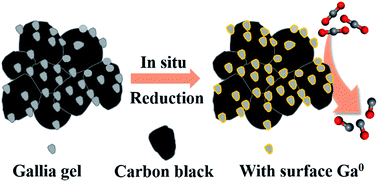
The electrochemical CO2 reduction reaction (CO2RR) powered by renewable electricity has emerged as a promising approach to alleviate global warming and energy depletion simultaneously. Notably, efficient catalysts containing Earth-abundant elements to achieve high CO2RR performance are in great demand for future applications. Herein, carbon-supported gallia gel nanoparticles were synthesized by precipitating gallium nitrate on carbon black in an ethanolic ammonia solution. Nano-sized gallia nanoparticles uniformly dispersed on the carbon support achieved a maximum CO faradaic efficiency of 77.0% at −0.71 V vs. the reversible hydrogen electrode (RHE) in CO2-saturated 0.1 M KHCO3 solution, showing a dramatic improvement compared to a bulk Ga electrode with only 24.2% CO faradaic efficiency at −0.80 V vs. RHE. X-ray photoelectron spectroscopy measurements revealed that surface Ga3+ species were reduced to metallic Ga when subjected to a negative potential during the CO2RR, indicative of the formation of oxide-derived active gallium sites. Control experiments further highlighted the necessity of close coalescence between the nano-sized gallia particles and the conductive carbon support. The present study underscores the feasibility of improving the CO2RR performance of Ga-related materials through nanostructuring of oxide-derived gallium catalysts.


 Please wait while we load your content...
Please wait while we load your content...