Energy-efficient 1.67 V single- and 0.90 V dual-electrolyte based overall water-electrolysis devices enabled by a ZIF-L derived acid–base bifunctional cobalt phosphide nanoarray†
Abstract
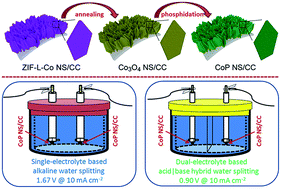
Zeolitic imidazolate frameworks (ZIFs) have been widely utilized as templates to construct hollow or porous nanostructures with superior catalytic properties for various electrocatalytic reactions. In this work, we reported that a leaf-like cobalt phosphide nanoarray topology assembled on carbon cloth (CoP NS/CC) built through annealing followed by phosphorization of the template precursor of the ZIF-L-Co nanosheet array on CC could serve as an efficient versatile catalyst for water electrolysis. This CoP NS/CC exhibits satisfactory HER, OER and full water-splitting efficiencies, with overpotentials of 90 mV and 310 mV and a voltage of 1.67 V needed to afford a current density of 10 mA cm−2, respectively. Also, this electrode shows an excellent and stable HER performance in 0.5 M H2SO4 with a low overpotential of 88.7 mV required to attain a current density of 10 mA cm−2. In this regard, a novel acid|base hybrid overall water-splitting electrolyzer with this CoP NS/CC serving as both the anode and cathode was further built, wherein cell voltages of just 0.735 (onset voltage) and 0.9 V were needed to afford overall current densities of 1 and 10 mA cm−2, respectively.


 Please wait while we load your content...
Please wait while we load your content...