A metal-ion-chelating organogel electrolyte for Le Chatelier depression of Mn3+ disproportionation of lithium manganese oxide spinel†
Abstract
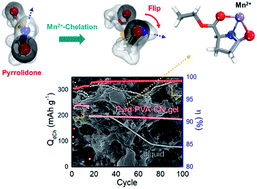
We present a metal-ion-chelating organogel electrolyte, thermally gelated within cells, to solve the problems triggered by metal dissolution from cathodes of lithium ion batteries. The organogel significantly improved the capacity retention of lithium manganese oxide spinel during cycling. The organogel mitigated metal deposition on anodes by capturing metal ions (anode protection). Interestingly, the organogel inhibited metal dissolution by keeping dissolved metal ions highly concentrated around the cathode surface (cathode protection by Le Chatelier's principle).


 Please wait while we load your content...
Please wait while we load your content...