Charge storage mechanism and degradation of P2-type sodium transition metal oxides in aqueous electrolytes†
Abstract
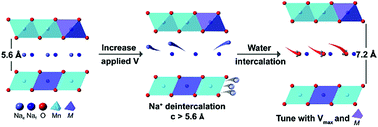
Few transition metal oxides exhibit sufficient stability for aqueous ion intercalation from neutral pH electrolytes for low-cost aqueous Na+ batteries and battery-type desalinators. P2 layered Na+ manganese-rich oxides have high theoretical capacities and voltages for Na+ storage and are extensively investigated for non-aqueous Na+ batteries. However, the charge storage mechanism and factors controlling interlayer chemistry and redox behavior of these materials in aqueous electrolytes have not been determined. Here, we take a significant step in establishing their aqueous electrochemical behavior by investigating a series of P2 oxides that exhibit a range of stability in water and ambient air: Na0.62Ni0.22Mn0.66Fe0.10O2 (NaNMFe), Na0.61Ni0.22Mn0.66Co0.10O2 (NaNMCo), Na0.64Ni0.22Mn0.66Cu0.11O2 (NaNMCu), and Na0.64Mn0.62Cu0.31O2 (NaMCu). Depending on the transition metal composition and potential, all materials exhibit significant irreversible Na+ loss during the first anodic cycle followed by water intercalation into the interlayer. The presence of water causes conversion into birnessite-like phases and microscopic exfoliation of the particles. The interlayer affinity for water is primarily driven by the Na+ content, which can be tuned by the transition metal composition and the maximum anodic potential during electrochemical cycling. The interlayer water affects the reversible capacity and cycling stability of the oxides, with the highest reversible capacity (∼40 mA h g−1 delivered in ∼30 minutes) obtained with NaNMCo. These results present the first studies on the structural effects of aqueous electrochemistry in P2 oxides, highlight the significant differences in the electrochemical behavior of P2 oxides in aqueous vs. non-aqueous electrolytes, and provide guidance on how to use the transition metal chemistry to tune their aqueous charge storage behavior.
- This article is part of the themed collection: Journal of Materials Chemistry A Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...