Enhanced electrocatalytic activity by chemical nitridation of two-dimensional titanium carbide MXene for hydrogen evolution†
Abstract
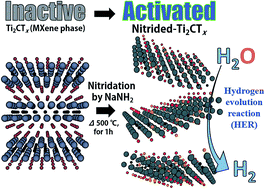
Developing active and stable electrocatalysts from Earth-abundant elements is the key to water splitting for hydrogen production through electrolysis. Here, we report a strategy to turn non-electrocatalytic Ti2CTx into an active electrocatalyst by the nitridation of two-dimensional (2D) titanium carbide MXene (Ti2CTx) nanosheets using sodium amide (NaNH2). The addition of NaNH2 results in the chemical bonding of Ti–Nx at 500 °C on the surface of Ti2CTx, which was designed as an efficient electrocatalytic material for the hydrogen evolution reaction (HER). When used as an electrocatalytic material for the HER, the nitrided-Ti2CTx (N-Ti2CTx) exhibited high activity with an overpotential of −215 mV vs. NHE for the hydrogen evolution reaction (HER) at 10 mA cm−2. These values are over three times smaller than those for pristine-Ti2CTx (−645 mV vs. NHE for the HER). The as-synthesized sample showed excellent durability under acidic (0.5 M H2SO4) conditions, indicating its robust catalytic activity towards the HER. The nitridation strategy implemented here could be extended to other 2D transition metal carbide electrocatalysts to improve their catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...